Nutrient Loading and Extreme Precipitation
Inadequate farming and community land use practices increase nutrient loading, in particular phosphorus loading. Overusing fertilizers, keeping livestock near water supplies, sewage discharges, and run-off are all common contributors to high nutrient loading. Heavy precipitation increases levels of contaminated runoff and nutrient loading in lakes and major waterways. Current vulnerabilities to extreme and sustained precipitation that encourage algal blooms, such as agricultural runoff and combined sewage overflows, will be amplified with more total and extreme precipitation.
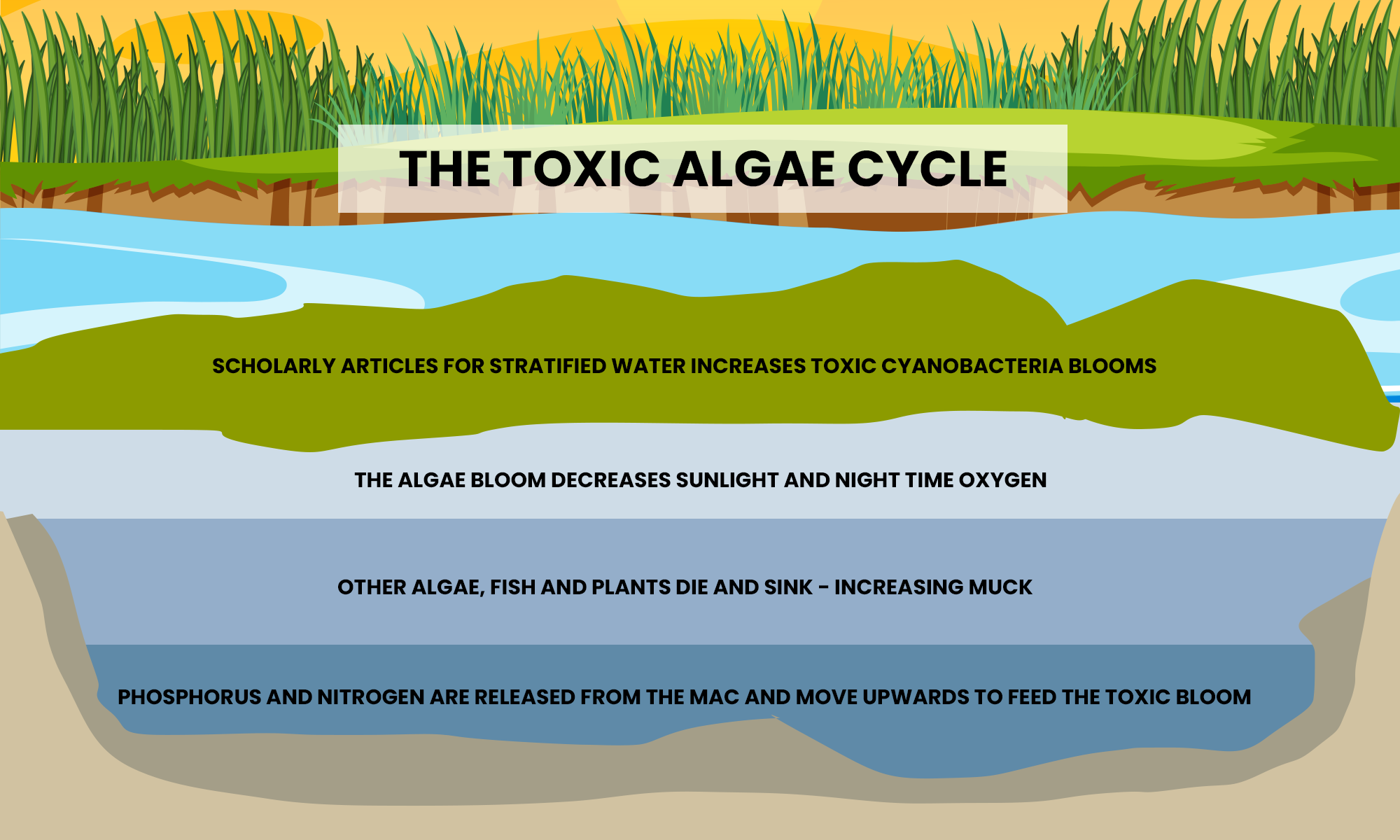
Fish and Hypoxia
Under normal conditions, algae are critical to the health of the Great Lakes ecosystem, providing the main source of energy that sustains many species of marine life. However, when algal blooms grow uncontrollably, they can create low-oxygen, hypoxic conditions. When excessive organic matter from algal blooms sinks into bottom waters, it decomposes and reduces dissolved oxygen concentrations. Warmer temperatures and stratified lake temperatures, that reduce vertical mixing, create the conditions for algae to grow out of control and cause oxygen-depleted “dead zones.” Massive fish kills result from these organisms being trapped in warm-temperature waters that are oxygen-depleted.